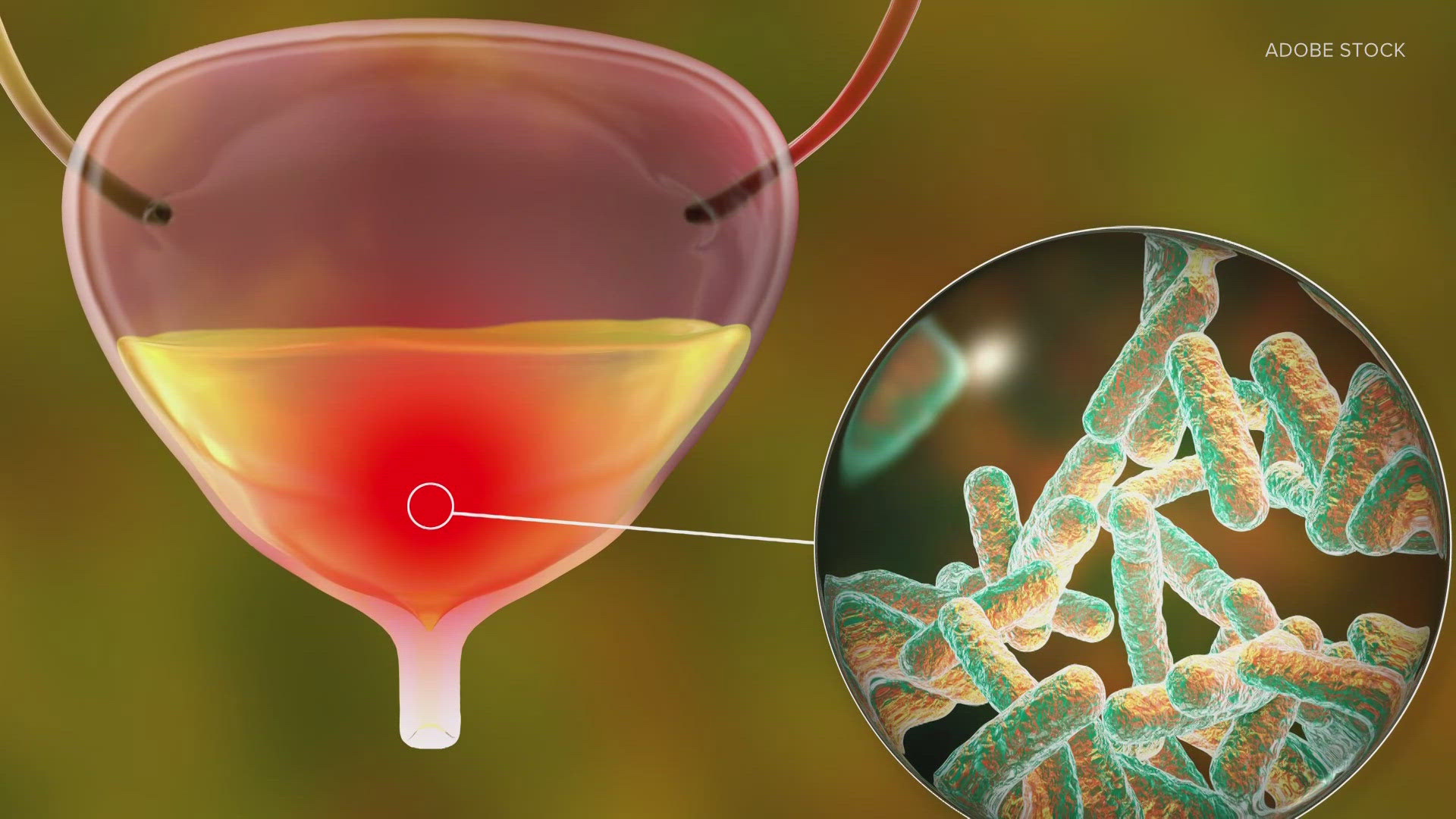
SEATTLE — For the first time in 20 years, the Food and Drug Administration (FDA) approved a new antibiotic to treat urinary tract infections in the U.S., easing concerns of antibiotic resistance.
A urinary tract infection, commonly referred to as UTIs for short, usually affects the urethra -- the part of the urinary system that moves urine out of the bladder. The bacterial infection can cause frequent, painful urination and in severe cases, it can lead to a kidney infection.
UTIs are very common, with half of all women enduring one in their lifetime.
"It tends to affect women more than men due to anatomy," said Dr. Reena Vasavada-Parikh, an OB-GYN based out of Swedish Hospital in Edmonds.
For decades, the antibiotic pivmecillinam -- under the brand name Pivya -- has been prescribed in Europe to treat what's known as uncomplicated UTIs, which are the kind that are confined to the urethra and bladder.
"It has slightly less efficacy than the ones we currently use, however, the chance of developing resistance is less," said Vasavada-Parikh.
In April, the FDA approved it for use in the U.S., though it's unclear when its manufacturer will have it available in the U.S. market.
But the addition of a new antibiotic to treat UTIs comes at a time when pathogens are increasingly at risk of becoming resistant to antibiotics. It's a relevant issue when it comes to UTIs, which the FDA said is one of the most frequent reasons for antibiotic use.
"Antibiotic resistance in everything is super important because once an organism develops a resistance to the antibiotic, it limits what we can use to treat it," Vasavada-Parikh said.
She added that she'll continue prescribing sulfa-based antibiotics typically used to treat UTIs but welcomed this additional option to help patients.
"To be honest, it's just another tool that we use," Vasavada-Parikh said. "It's just nice to have something else that we can use to treat."